A vendor audit rarely fails because of a single missing document. They fail because information is fragmented, decisions are undocumented, and evidence is scattered across emails, folders, and spreadsheets.
In regulated and operationally complex industries, vendor audits are the final checkpoint of everything that happened before: how vendors were selected, how certifications were tracked, how issues were resolved, and whether corrective actions actually closed the loop.
That’s why vendor audits should not be treated as periodic events. They are the visible result of how well vendor data, documentation, and decisions are managed over time.
This article explains what a vendor audit is, why it matters across regulated industries, and how companies can move from reactive audit preparation to continuous audit readiness.
What Is a Vendor Audit?
A vendor audit is a structured evaluation of a third-party supplier to verify compliance, quality standards, operational controls, and regulatory requirements. Its goal is to ensure that vendors consistently meet the expectations defined by contracts, regulations, and internal policies.
Unlike internal audits, which assess an organization’s own processes and controls, vendor audits focus externally. They examine how suppliers operate, how they manage risks, and whether their processes align with the buyer’s regulatory and quality obligations.
Vendor audits are closely related to supplier audits. While the terms are often used interchangeably, “vendor audit” is commonly used in procurement, compliance, and regulatory contexts, while “supplier audit” appears more frequently in manufacturing and quality management systems.
Organizations audit vendors for several reasons, like:
- Risk management, to identify operational, financial, or compliance risks before they escalate;
- Regulatory compliance, especially in industries subject to inspections and external audits;
- Quality assurance, ensuring materials, services, or components meet defined standards;
- Business continuity, reducing dependency risks across the supply chain.
Why Vendor Audits Matter
Vendor audits play a direct role in regulatory exposure, product quality, and organizational reputation. In many industries, a compliance failure at the vendor level is treated as a failure of the contracting company itself.
From a regulatory standpoint, authorities increasingly expect organizations to demonstrate control over their extended supply chain. In pharmaceutical, manufacturing, logistics, and packaging environments, regulators do not accept “vendor responsibility” as a defense.
Companies must show documented oversight, qualification, and follow-up.
Quality and safety risks are equally critical. A single non-compliant supplier can compromise raw materials, packaging integrity, or process reliability, leading to recalls, production shutdowns, or safety incidents. Vendor audits are often the only formal mechanism to identify some risks before they impact operations.
There is also a strong dependency factor. As supply chains become more specialized, organizations rely on fewer, more critical vendors. This concentration increases exposure and makes consistent vendor evaluation essential, not optional.
Finally, vendor audits affect brand and reputation. Audit findings, enforcement actions, or public recalls tied to supplier failures can damage trust with customers, partners, and regulators alike.
For this reason, vendor audits are tightly connected to broader regulatory audits and quality inspections. In sectors like pharmaceuticals and advanced manufacturing, vendor audits are often reviewed as part of FDA, ISO, or other regulatory assessments, making audit readiness a continuous operational requirement rather than a seasonal activity.
Types of Vendor Audits
Vendor audits can take different forms depending on risk level, industry, and the supplier’s criticality to the operation. Below are the main audit types and how to structure a consistent vendor audit program.
Compliance and Regulatory Audits
Compliance and regulatory audits assess whether a vendor meets legal, regulatory, and certification requirements. They are common in regulated industries such as pharmaceuticals, food, logistics, and manufacturing.
The focus is on formal requirements, including valid certifications, operating licenses, legal registrations, and compliance with ISO standards, FDA regulations, or local regulatory frameworks. Failures in this type of audit can result in penalties, supply chain disruptions, or even legal exposure for the contracting company.
How AnyDB supports this type of audit
AnyDB allows teams to create customized compliance audit templates with structured fields for expiration dates, regulatory bodies, approval status, and mandatory document uploads.
ISO certificates, legal authorizations, and other regulatory documents are attached directly to the vendor or audit record, keeping data and evidence in one place.
Quality Audits
Quality audits evaluate whether a vendor meets technical, operational, and performance standards. They are especially critical in sensitive supply chains, such as pharmaceuticals and packaging materials, where deviations can impact safety, efficacy, or regulatory compliance.
In this context, quality is not an abstract concept. It is tied to specific products, raw materials, batches, and production processes. That is why quality audits require context and historical traceability.
How AnyDB supports this type of audit
With AnyDB’s object-based data model, an audit record can be directly linked to a specific raw material, Bill of Materials (BOM) item, or supplied product.
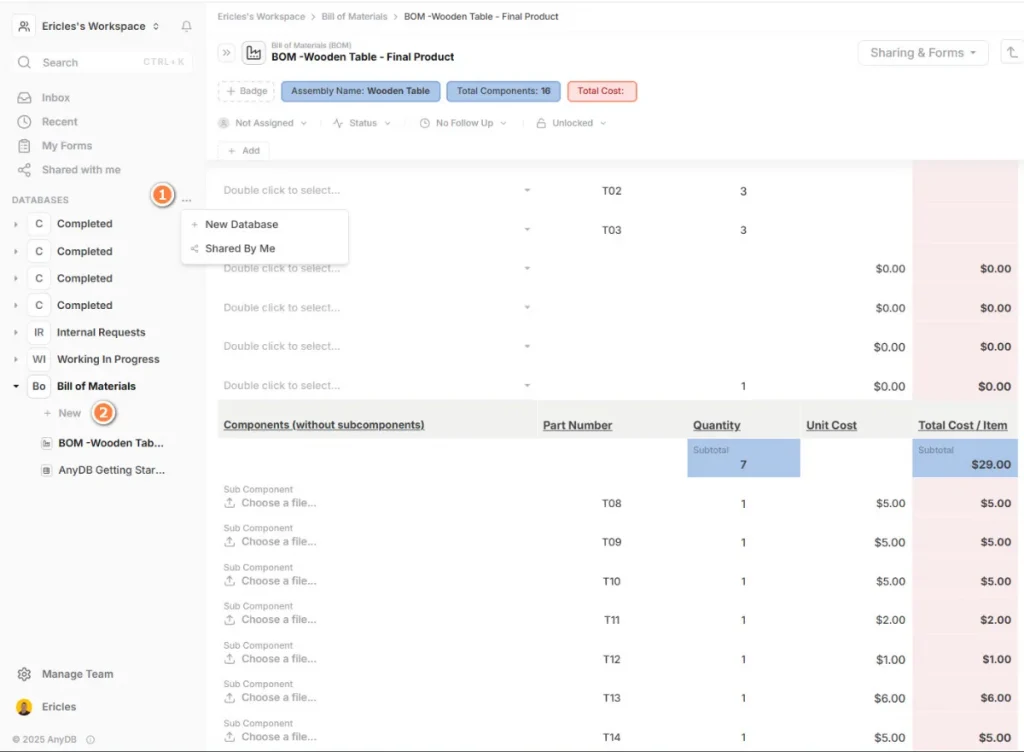
This enables auditors to navigate from the audited item to the vendor’s complete history, including past audits, non-conformities, and related corrective actions.
Operational and Process Audits
Operational and process audits evaluate how vendors perform their daily activities. Unlike regulatory audits, the focus here is on delivery, performance, and operational controls.
These audits assess whether deadlines are met, processes are consistent, internal controls are effective, and the vendor can sustain the agreed service level over time.
In complex supply chains, operational failures are often the first signal of broader risks, such as disruptions, cost increases, or quality degradation.
How To Conduct a Vendor Audit?
Conducting a vendor audit requires method, criteria, and traceability. Below is a step-by-step approach:
- Audit Planning and Scope Definition
Not all vendors require the same level of audit depth. Factors such as product criticality, regulatory impact, incident history, and operational dependency help determine which vendors should be audited and how frequently. This phase also defines the audit criteria, including applicable standards, contractual requirements, internal policies, and expected performance indicators.
- Vendor Qualification and Pre-Audit Review
During the pre-audit review, validate supplier documentation such as contracts, certifications, licenses, quality records, and prior compliance evidence. In regulated industries, vendor qualification for packaging materials or critical inputs is especially sensitive. Missing documents or lack of historical traceability already indicate risk before the audit begins.
- Audit Execution and Evidence Collection
Audits can be conducted on-site or remotely, depending on context and risk level. During execution, auditors conduct interviews, verify documents, analyze processes, and observe operations in practice. On-site audits take place on factory floors, distribution centers, or operational areas, where fast and accurate evidence capture is essential. AnyDB supports mobile access and real-time updates to record observations and take photos of non-compliance issues.
- Findings, CAPA, and Follow-Up
After an audit, findings are typically classified as conformities, observations, or non-conformities. When deviations are identified, corrective and preventive actions, commonly known asCAPA, come into play. The real challenge is not logging the issue, but ensuring it is addressed, tracked, and closed in a verifiable way. In AnyDB, an audit finding can generate CAPA records linked as child objects to the main audit record. Each CAPA can be assigned to an owner, with defined deadlines and status.
Vendor Audit Checklist
Checklists remain a fundamental audit tool, considering they are not treated as static documents. A vendor audit checklist typically includes:
- Valid certifications and licenses;
- Insurance policies and legal documentation;
- Contracts and service level agreements (SLAs);
- Quality and inspection records;
- Incident and deviation history;
- Open and closed corrective and preventive actions.
The issue is not the checklist itself, but how it is maintained. Without a structured system, checklists quickly become outdated, lose context, and fail to reflect reality.
To support this step, AnyDB provides a structured vendor audit checklist template that can be adapted to different industries and risk levels:
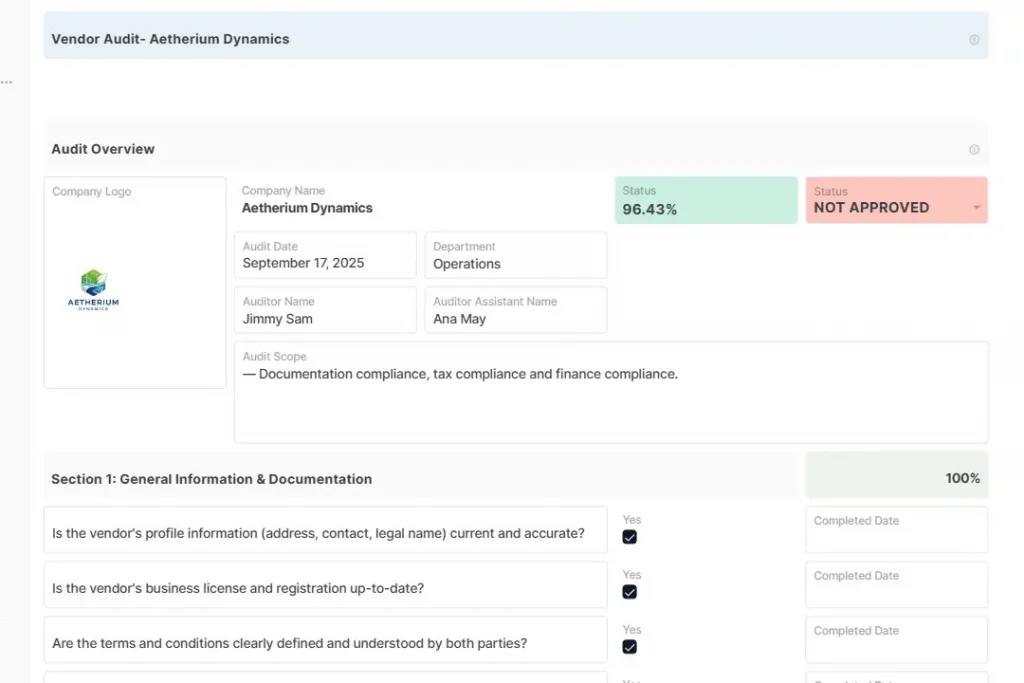
How AnyDB Supports Continuous Vendor Audit Readiness
Successful audits reflect how data, documents, and decisions have been organized over time. This is where AnyDB stands apart by making audit readiness a natural part of daily operations. Here’s how:
- Continuous, not seasonal, audits: AnyDB is designed to support vendor audits on an ongoing basis, eliminating last-minute evidence gathering and audit-season stress.
- Structured vendor records: Each vendor record includes defined fields for scope, qualification status, risks, and internal owners, creating a single source of truth.
- Documents, contracts, and certifications in the right context: They are linked to the vendor, audit type, and relevant materials or processes, ensuring clear and traceable evidence.
- Full audit logs and version history: Every change is recorded, making it possible to prove when a document was added, who verified it, and which version was valid at any point in time.
- Clear linkage between audits, findings, and CAPA: Non-conformities generate corrective and preventive actions directly connected to the original audit, forming a logical and fully auditable chain.
- Role-based access control: Permissions are defined by role, ensuring only authorized users can edit, approve, or close critical records.
- Historical traceability by default: Decisions, documents, and actions remain recorded over time, enabling audits based on facts rather than last-minute reconstructions.
Frequently Asked Questions About Vendor Audit
Learn more about vendor audits below:
Audit frequency depends on risk level, supply criticality, regulatory requirements, and vendor history. Critical or regulated vendors typically require more frequent audits, while lower-risk suppliers may be reviewed on longer cycles.
Common requirements include contracts, certifications, licenses, insurance policies, quality records, incident history, and evidence of corrective actions. Regulated industries may impose additional documentation requirements.
Well-prepared organizations keep data and documents organized, current, and linked to the proper context. This includes historical records, clear audit trails, and continuous CAPA follow-up, rather than last-minute preparation.
Effective audit trails require structured records of selection criteria, comparative evaluations, approvals, and justifications. When this information is linked to the vendor record and protected by version history, decisions remain auditable even years later.
Tracking requires visibility into expiration dates, verification of owners, attached documents, and change history. Structured systems enable alerts, traceability, and clear evidence that certifications were valid and verified at the right time.
What is AnyDB?
AnyDB is a unified, customizable data store designed to streamline and empower your entire organization. Effortlessly store, organize, and share custom business data to drive both internal and external operations across teams. Think of it as spreadsheets on steroids.Perfect for Sales, Marketing, Operations, HR, and beyond. Discover AnyDB





