Some may think managing an ISO audit has become more complex. But in reality, the main challenges come from teams working with disconnected spreadsheets, documents scattered across multiple folders, and parallel conversations that make it hard to track what really matters.
You can probably already imagine the consequences: missing evidence during audits, unclear responsibilities, difficulty tracking non-conformances and corrective actions, and lack of real-time visibility into the process.
On the other hand, structured record systems bring standardization, traceability, and the ability to link audits, NCRs, evidence, KPIs, and corrective actions within a single environment. This shift is exactly what makes ISO audit management more robust and scalable. Learn more in the sections below.
What Is an ISO Audit?
An ISO Audit is the process of verifying whether an organization’s management systems comply with standards such as ISO 9001 (quality), ISO 14001 (environmental management), and other complementary frameworks.
It can be conducted internally or by a certification body and assesses whether processes, controls, indicators, and records are properly implemented. Key elements are evaluated to determine if the system works as designed and supports continuous improvement practices, including:
- Documentation
- Operational evidence
- Performance records
- Corrective actions
- Non-conformance reports
How an ISO Audit Fits Into the Compliance Cycle
Inside the compliance cycle, the audit functions as an essential mechanism. A mature organization typically follows this sequence:
- Internal audit identifies adherence, gaps, and opportunities
- Findings are recorded, categorized, and prioritized
- NCR formalizes the non-conformance
- Corrective action defines root cause, plan, and ownership
- Verification confirms whether the action resolved the issue
- External certification validates and consolidates the entire cycle
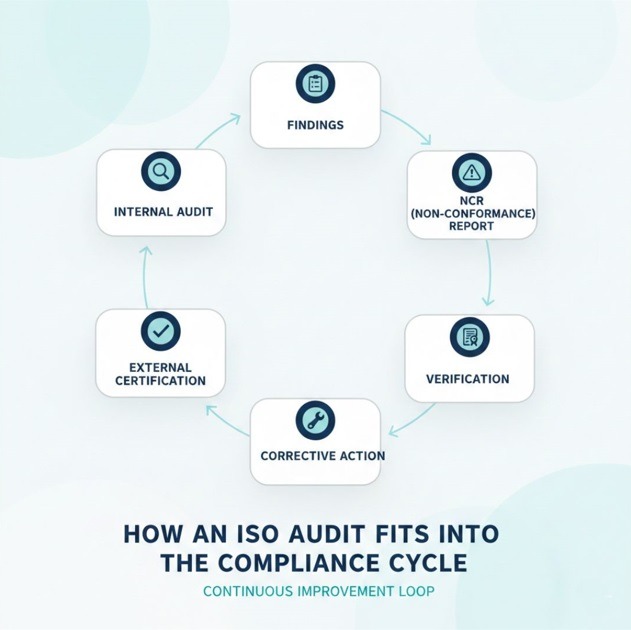
This cycle creates a continuous improvement loop that ensures consistency, reduces risks, and prepares the organization for future audits with greater predictability.
Key Components of an ISO Audit
An effective ISO Audit management relies on a structured analysis of the management system. Each step provides visibility into compliance, risks, and opportunities for improvement. Below is a technical breakdown of the main components:
Audit Criteria and Scope
The auditor defines which standards, clauses, and processes will be evaluated. The scope sets the boundaries for areas, units, documents, and activities included in the audit.
Documentation Review
The first check focuses on formal records. Policies, procedures, manuals, reports, and performance indicators are examined to confirm whether they meet the standard’s requirements.
Process Walkthroughs
The auditor observes and follows processes to validate that documented procedures are actually executed. Interviews, observations, and operational analyses are part of this stage.
Evidence Collection
All conclusions must be based on evidence. This includes records, logs, forms, measurements, traceability, attachments, and information gathered during the walkthrough.
Risk and Compliance Assessment
The audit evaluates risks related to control failures, operational impacts, and points that could compromise ISO compliance.
Audit Findings and Classification
Findings are recorded and classified as minor or major depending on their impact on standard compliance.
Non-Conformance Reporting
When a requirement is not met, the auditor issues a formal NCR, describing the deviation and its immediate cause.
Root-Cause Analysis
The organization investigates the root cause of the non-conformance to prevent recurrence. Tools like Ishikawa diagrams and the “5 Whys” method are commonly used.
Corrective and Preventive Actions (CAPA)
Based on the analysis, an action plan is created with assigned responsibilities, deadlines, and effectiveness criteria. Preventive actions may also be recommended for identified risks.
Follow-up and Closure
The auditor verifies whether the corrective action has been implemented and effectively resolved the issue. Only then is the non-conformance formally closed.
Internal vs External ISO Audits: What’s the Difference?
ISO audits may follow similar structures, but they serve different purposes in the compliance lifecycle. Internal audits strengthen processes; external audits validate them.
Understanding how internal and external audits complement each other helps teams prepare better, reduce surprises, and maintain consistent certification readiness.
Below is a streamlined comparison to help teams quickly identify what changes between the two:
| Aspect | Internal ISO Audit | External ISO Audit |
| Who performs it | Internal teams or hired consultants | Accredited certification bodies |
| Primary purpose | Identify gaps, risks, and improvement opportunities | Assess compliance against ISO standards and grant/maintain certification |
| Depth & flexibility | More detailed, customizable, and operational | More formal, structured, and guided by strict certification protocols |
| Focus areas | Process understanding, evidence checking, readiness evaluation | Conformance validation, non-conformance classification, certification decision |
| Outputs | Findings, internal NCRs, corrective actions, improvement insights | Official report, minor/major non-conformities, improvement opportunities |
| Impact | Drives continuous improvement | Determines certification status and external credibility |
ISO Audit Checklist
A checklist helps your team organize evidence, avoid gaps, and ensure consistency throughout the compliance cycle. Here’s a clear, scannable example:
- Policies and Procedures
- Training Records
- Quality Objectives and Internal Goals
- Process Documentation and Operational Flows
- Environmental Metrics (for ISO 14001)
- Equipment Calibration and Maintenance Records
- Up-to-Date NCRs and CAPAs
- Supplier Evaluations and Performance
- Audit Trail Evidence and Activity Logs
- Management Review Records and Executive Decisions
The ISO Audit Cycle Explained
Understanding each stage of an ISO audits helps teams prepare systematically and demonstrate consistent control over their management systems.
- Planning & Scoping
The audit begins with defining scope, objectives, criteria, timeline, and required resources. Auditors determine which processes, departments, and records will be evaluated.
- Document Review
Policies, procedures, manuals, risk assessments, indicators, NCR logs, and past audit records are examined to ensure alignment with ISO requirements before on-site activities start.
- Field Audit
Auditors interview personnel, observe processes, verify controls in practice, inspect records, and look for evidence of conformity. This is where real-world implementation is validated.
- Findings Report
All observations are categorized as conformities, opportunities for improvement, or nonconformities (major/minor), including objective evidence for each.
- Corrective Actions
For nonconformities, organizations must identify root causes, implement corrective measures, assign owners, and establish deadlines.
- Follow-up Audit
Auditors review the effectiveness of corrective actions, verify closure evidence, and ensure that issues were resolved without recurrence.
- Continuous Improvement
The cycle ends and restarts with systemic learning, preventive action, and optimization of processes, reinforcing ISO’s fundamental principle of ongoing improvement.
Managing ISO Audits With AnyDB
Managing ISO audits becomes easier when audit plans, evidence, and corrective actions live in a structured, relational environment. AnyDB provides such an ecosystem not as a traditional QMS platform, but as a flexible data system designed to organize compliance information with precision.
AnyDB stores structured business records with support for linked relationships, allowing audits, NCRs, CAPAs, suppliers, and documentation to connect naturally. Each record maintains its audit trail and version history, ensuring the traceability ISO auditors expect.
The platform combines a spreadsheet-like interface with a relational database structure, giving compliance teams the familiarity of tables with the rigor of connected records.
Permissions can be set down to the cell or record level, enabling organizations to share a finding, an NCR, or a single evidence document with auditors without exposing unrelated information (source: AnyDB brand and product documentation).
Templates for ISO 9001, ISO 14001, internal audits, NCRs, and CAPA workflows provide a reliable foundation:
Within an audit program, teams can store:
- Audit plans and criteria
- Process evidence
- Findings and objective evidence
- Linked NCRs and CAPAs
- Closure actions and verification notes
AnyDB also supports automated notifications and evidence collection through integrations with Make and Zapier, enabling reminders, follow-up tracking, and centralized documentation. The result is a flexible, connected environment that helps teams remain audit-ready year-round, not just during audit season.
When to Replace Spreadsheets With ISO Audit Software
Spreadsheets work for early-stage compliance efforts, but they quickly become a bottleneck as audit complexity grows. Certain operational triggers signal it’s time to migrate to a structured audit platform:
- Too many NCRs to track manually: Corrective actions get lost, duplicated, or delayed.
- Missing evidence or attachments: Files live in emails, shared drives, or personal folders.
- Version conflicts: Multiple team members editing parallel files creates inconsistencies.
- Hard to maintain audit history: Spreadsheets lack reliable audit trails and version logs.
- Difficult cross-department collaboration: Sharing sensitive data across teams becomes risky.
- No automation or reminders: Deadlines, corrective action reviews, and follow-ups depend on manual tracking.
Start managing your ISO audits in AnyDB: build your first audit workflow for free.
FAQs About ISO Audit
ISO audits can seem complex, but most questions come down to understanding structure, criteria, and preparation. Below are concise, practical answers to the essentials.
Scope, criteria, documented information, on-site evaluation, findings, and corrective action requirements.
Planning, document review, field audit, reporting, corrective actions, follow-up, and continuous improvement.
The specific clauses of the ISO standard being applied, plus internal procedures and regulatory requirements.
Organize records, verify procedures align with practice, correct known gaps, brief teams, and prepare evidence.
Internal audits are conducted by the organization (or hired auditors); external audits are performed by certification bodies.
Process reviews, evidence checks, conformity assessment, interviews, findings, and documented follow-up actions.
What is AnyDB?
AnyDB is a unified, customizable data store designed to streamline and empower your entire organization. Effortlessly store, organize, and share custom business data to drive both internal and external operations across teams. Think of it as spreadsheets on steroids.Perfect for Sales, Marketing, Operations, HR, and beyond. Discover AnyDB





